| Identification | Back Directory | [Name]
2-[[4-[(7-Chloroquinolin-4-yl)amino]pentyl](ethyl)amino]ethanol | [CAS]
118-42-3 | [Synonyms]
C07043
win1258
plaquenil
Oxychloroquine
hydroxychloroquine
2-((4-((7-chloro-4-quinolyl)amino)pentyl)ethylamino)-ethano
2-((4-((7-chloro-4-quinolyl)amino)pentyl)ethylamino)ethanol
2-[[4-[(7-Chloroquinolin-4-yl)amino]pentyl](ethyl)amino]ethanol
Ethanol, 2-[[4-[(7-chloro-4-quinolinyl)amino]pentyl]ethylamino]-
7-chloro-4-(5-(n-ethyl-n-2-hydroxyethylamino)-2-pentyl)aminoquinoline
7-chloro-4-(4-(n-ethyl-n-beta-hydroxyethylamino)-1-methylbutylamino)quinolin | [EINECS(EC#)]
204-249-8 | [Molecular Formula]
C18H26ClN3O | [MDL Number]
MFCD00242707 | [MOL File]
118-42-3.mol | [Molecular Weight]
335.88 |
| Hazard Information | Back Directory | [Definition]
ChEBI: An aminoquinoline that is chloroquine in which one of the N-ethyl groups is hydroxylated at position 2. An antimalarial with properties similar to chloroquine that acts against erythrocytic forms of malarial parasites, it is mainly used
s the sulfate salt for the treatment of lupus erythematosus, rheumatoid arthritis, and light-sensitive skin eruptions. | [Originator]
Plaquenil,Winthrop,US,1956 | [Uses]
Antimalarial; suppressant
(lupus erythematosus). | [Indications]
Hydroxychloroquine (Plaquenil), like chloroquine, is a
4-aminoquinoline derivative used for the suppressive
and acute treatment of malaria. It also has been used for
rheumatoid arthritis and discoid and systemic lupus erythematosus.
Hydroxychloroquine has not been proved
to be more effective than chloroquine. Adverse reactions
associated with its use are similar to those described
for chloroquine.The drug should not be used in
patients with psoriasis or porphyria, since it may exacerbate
these conditions. | [Manufacturing Process]
A mixture of 323 grams of 1-chloro-4-pentanone, 480 grams of N-ethyl-N-2-
hydroxyethylamine and 400 grams of sodium chloride (to aid in subsequent
filtration) in 1.3 liters of xylene was heated with stirring on a steam bath for
two hours and then refluxed for three hours. After standing overnight, the
mixture was filtered and the filter cake washed with xylene. The filtrate was
fractionally distilled, yielding 207.3 grams of a fraction distilling at 89° to
90°C at 0.35 mm; nD25 = 1.4600. This fraction, 1-(N-ethyl-N-2-
hydroxyethylamino)-4-pentanone, was used in the next step of the synthesis.
A sample of the fraction was further purified by distillation through a column
and gave an analytically pure sample of 1-(N-ethyl-N-2-hydroxyethylamino)-
4-pentanone, boiling at 85° to 87°C at 0.4 mm.
The 1-(N-ethyl-N-2-hydroxyethylamino)-4-pentanone from above (284.2
grams) was dissolved in 300 grams of 28% ammoniacal methanol and
reduced catalytically with Raney nickel (at an initial pressure of 1,000 pounds)
at room temperature. After 24 hours the catalyst was filtered off and the
product distilled in vacuo through a column, yielding 254 grams of a fraction
distilling at 88.5° to 96°C at 0.3 mm and comprising mainly 5-(N-ethyl-N-2-
hydroxyethylamino)-2-pentylamine. An analytical sample of this fraction
distilled at 93°C at 0.6 mm.
A mixture of 90 grams of 4,7-dichloroquinoline, 90 grams of phenol, 1 gram
of potassium iodide and 132 grams of 5-(N-ethyl-N-2-hydroxyethylamino)-2-
pentylamine from above was heated with stirring for 13 hours at 125° to
130°C. Methanol (1.9 liters) was added and the the mixture was filtered with
charcoal. The filtrate was treated with 270 cc of a solution of 100 grams of
phosphoric acid in 300 cc of methanol. The walls of the flask containing the
filtrate were scratched with a glass rod and the mixture was allowed to stand
for two days. The solid was filtered off, washed with methanol and dried,
yielding 101 grams of crude 7-chloro-4-[5-(N-ethyl-N-2-hydroxyethylamino)-
2-pentyl]aminoquinoline diphosphate, MP 155° to 156°C.
Additional quinoline diphosphate was obtained as a gummy mass from the
filtrate by concentrating the latter to about half its volume and adding
acetone. The crude gummy diphosphate was dissolved in water, basified with
ammonium hydroxide and the resulting liberated basic quinoline extracted
with chloroform. After removal of the chloroform by distillation, the residue
was dissolved in ether and crystallization was induced by scratching the walls
of the flask with the glass rod. About 30 grams of the crude quinoline base,
melting at 77° to 82°C, separated. Recrystallization of this material from
ethylene dichloride or ethyl acetate yielded the purified 7-chloro-4-[5-(N�ethyl-N-2-hydroxyethylamino)2-pentyl] aminoquinoline, MP 89° to 91°C.
The base may then be dissolved in ethanol and precipitated as the sulfate by
reaction with an equimolar quantity of sulfuric acid. | [Therapeutic Function]
Antimalarial | [Mechanism of action]
Hydroxychloroquine, like chloroquine, is also used for treating acute forms of malaria
caused by P. vivax, P. malariae, P. ovale, and also sensitive forms of P. falciparum. It is
also effective and safe like chloroquine, although it does not have obvious advantages. The
only advantage is that it is somewhat better tolerated. Its use is somewhat more limited
than chloroquine. Synonyms of this drug are plaquenil, quensyl, toremonil, and others. | [Clinical Use]
Hydroxychloroquine is approved for the treatment
of both systemic and cutaneous lupus erythematosus.
Both chloroquine and quinacrine (Atabrine) are also effective
in this skin disease. Low-dose chloroquine is
used for the therapy of porphyria cutanea tarda in patients
in whom phlebotomy has failed or is contraindicated.
Other skin diseases in which the drugs are useful
(after sunscreens and avoidance of sun exposure) include
polymorphous light eruption and solar urticaria. | [Synthesis]
Hydroxychloroquine, 7-chloro-4-[4-[ethyl(2-hydroxyethyl)amino]-
1-methylbutylamino]quinoline (37.1.1.19), is made by a scheme similar to that of making
chloroquine. Reacting 1-chloro-4-pentanone with 2-ethylaminoethanol gives the corre�sponding aminoketone (37.1.1.17), which undergoes reductive amination in conditions
analogous to those described above, making 4-[ethyl(2-hydroxyethyl)amino]-1-methyl�butylamine (37.1.1.18). Reacting this with 4,7-dichlroquinoline (37.1.1.1) makes the
desired hydroxychloroquine.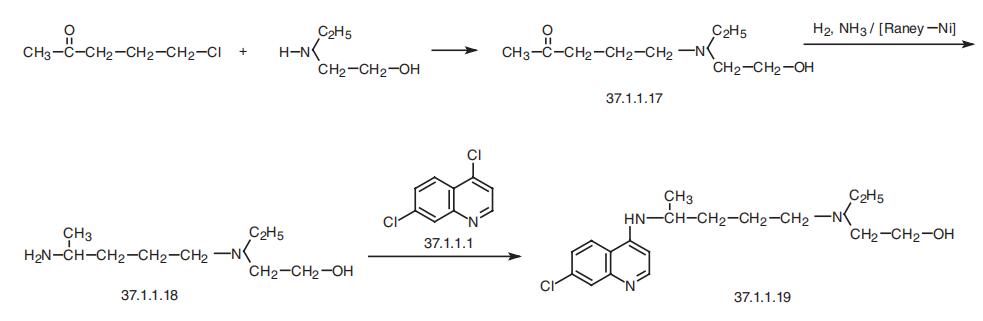
|
| Questions And Answer | Back Directory | [description]
Hydroxychloroquine is a synthetic antimalarial agent which can also inhibit Toll-like receptor 7/9 (TLR7/9) signaling. Hydroxychloroquine is efficiently inhibits SARS-CoV-2 infection in vitro.
Hydroxychloroquine, an analogue of chloroquine, was developed in 1946. Hydroxychloroquine and chloroquine are FDA-approved to treat or prevent malaria. Hydroxychloroquine is also FDA-approved to treat autoimmune conditions such as chronic discoid lupus erythematosus, systemic lupus erythematosus in adults, and rheumatoid arthritis.
Hydroxychloroquine is often taken in combination with other drugs such as methotrexate. | [Application in Particular Diseases]
In Rheumatic Arthritis:
Hydroxychloroquine lacks the myelosuppressive, hepatic, and renal toxicities seen with some other DMARDs, which simplifies monitoring. Its onset may be delayed for up to 6 weeks, but the drug should not be considered a therapeutic failure until after 6 months of therapy with no response.
Short-term toxicities include GI (nausea, vomiting, diarrhea), ocular (accommodation defects, benign corneal deposits, blurred vision, scotomas, night blindness, preretinopathy), dermatologic (rash, alopecia, skin pigmentation), and neurologic (headache, vertigo, insomnia) effects. Periodic ophthalmologic examinations are necessary for early detection of reversible retinal toxicity.
|
|
|